GROUP 7
I) GENERAL PROPERTIES OF THE GROUP 7 ELEMENTS
The elements of Group 7 are called the halogens:
Fluorine – F - [He]2s2 2p5
Chlorine - Cl - [Ne]3s2 3p5
Bromine – Br - [Ar]4s2 3d10 4p5
Iodine – I - [Kr]5s2 4d10 5p5
Astatine – At - [Xe]6s2 4f14 5d10 6p5
All the isotopes of astatine rapidly decay into other elements, so knowledge of its properties is not required. Fluorine’s small size and high electronegativity give it some anomalous properties.
The halogens are non-metallic elements with very similar properties. Their reactivity decreases down the group. Their characteristics are caused by the outermost 7 electrons. This causes them to regularly have oxidation states of -1 although other oxidation states do exist, especially for chlorine.
In compounds, a halogen atom increases its share of electrons from seven to eight (a full outer shell) by either gaining an electron to form a halide ion in ionic compounds, or sharing an electron in a single covalent bond.
The halogens in their elemental state form covalent diatomic molecules. Fluorine, chlorine and bromine are poisonous. As atomic number increases, their melting and boiling points increase. This is due to stronger van der Waals forces as the number of electrons present in a molecule increases.
Increase in the number of electrons increases the probability of the electron density being unbalanced on the molecule at any time. Also a greater difference in charge shown across the molecule makes the forces stronger.
-
Fluorine is a pale yellow gas
-
Chlorine is a greenish-yellow gas
-
Bromine is a dark red liquid, however is volatile and therefore often turns into a brown dense vapour
-
Iodine is a purple-black solid, which sublimes into a purple vapour when heated.
The halogens are all oxidising agents affected most by their electronegativities – fluorine is the most electronegative element in the periodic table and the electronegativities decrease down the group.
In many reactions the halogen will gain an electron in its outer shell to form a negative ion. This is easier the smaller the atom, as the outer shell is nearer to the attractive force of the nucleus and there are fewer inner shells providing shielding.
To summarise:
-
They all behave chemically in a similar way – form diatomic molecules, form 1- ions when they form halide salts with metals.
-
Reactivity decreases down the group as it is more difficult to gain an outer shell electron the further from the nucleus.
-
In compounds, the halogens increase the number of electrons in their outer shell to eight by sharing electrons or gaining them to form ions.
-
Their oxidising ability (ability to steal electrons) decreases down the group.
The halogens ability to form covalent and ionic compounds makes them very useful. Some of the uses are:
-
As a germicide - chlorinating water (to kill bacteria)
-
Make plastics such as PVC
-
Make non-stick (Teflon) PTFE – polytetrafluoroethene
-
Fluoridation of water to prevent tooth decay
-
Etch glass – hydrofluoric acid
-
Propellant in aerosols and fire extinguishers
-
Silver bromide is used in photography
-
Iodine is used as an antiseptic and is important in the diet.
II) THE REACTIVITY OF THE HALOGENS: DISPLACEMENT REACTIONS
The more negative the electron affinity of an element, the greater ease it can capture an electron and the greater the oxidising power.
The oxidising power of the halogens decreases down the group as it becomes more difficult for an electron to be captured the further the outer shell is from the nucleus.
As the oxidising power changes down the group, halogens can displace each other in reactions. The more oxidising halogen will take the electron from another halide ion, causing it to become a halide ion itself and rendering the less oxidising halogen a free radical, which will combine to form a halogen diatomic molecule.
Chlorine is the most oxidising halogen so it will displace bromide and iodide ions from a compound:
Cl2 (aq) + 2Br- (aq) à 2Cl- (aq) + Br2 (aq)
Cl2 (aq) + 2I- (aq) à 2Cl- (aq) + I2 (aq)
Iodine will not displace any of other halogens from a compound. Bromine will displace iodide ions but not chloride ions.
Halide ion solutions are all colourless and dilute solutions of the halogens can appear colourless so it is difficult to tell whether a reaction has taken place. Cyclohexane is usually added as a solvent to make the halogens more soluble, so they can be seen in the reaction.
-
Chlorine is a pale yellow colour dissolved in cyclohexane
-
Chlorine is clear dissolved in water
-
Bromine is an orange colour dissolved in cyclohexane
-
Bromine is pale orange dissolved in water
-
Iodine is purple in cyclohexane
-
Iodine is brown in water.
The colour observed tells us which halogen was displaced from the compound. By comparing this to the colour before the halide solution is added, we can tell whether a reaction has taken place.
A test for each halide in a solution can be completed using silver nitrate solution.
-
A white precipitate indicates the presence of chloride ions Cl- (aq)
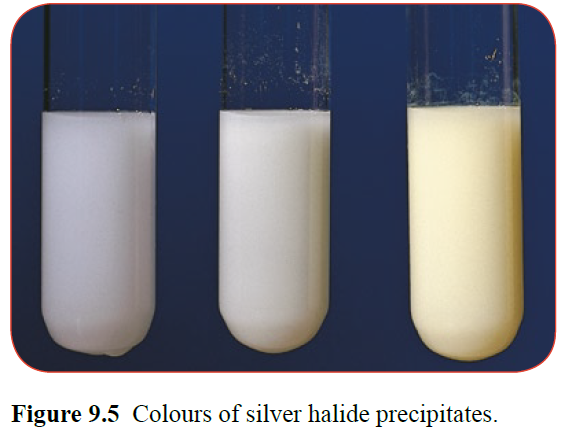
-
A cream precipitate indicates the presence of bromide ions Br- (aq)
-
A white precipitate indicates the presence of iodide ions I- (aq)
Identification by colour is not completely reliable. A further test is to add different concentrations of ammonia:
-
White silver chloride dissolves in dilute ammonia, forming a colourless solution
-
Cream silver bromide dissolves in concentrated ammonia, forming a colourless solution
-
Yellow silver iodide does not dissolve in concentrated aqueous ammonia.
III) DISPROPORTIONATION REACTIONS OF CHLORINE
The way in which chlorine reacts with aqueous sodium hydroxide is dependent on temperature.
With cold dilute sodium hydroxide, a mixture of chloride (Cl-) and chlorate (I) (ClO-) ions is formed:
Cl2 (g) + 2NaOH (aq) à NaCl (aq) + NaClO (aq) + H2O (l)
This reaction is an example of a disproportionation, as chlorine is both oxidised and reduced in the reaction. An ionic equation shows this:
This reaction is used to commercially produce bleach, which is known as sodium hypochlorite (sodium chlorate).
Chlorine is also used to treat drinking water. Disproportionation occurs again to form hydrochloric acid and chloric (I) acid:
Cl2 (g) + H2O (l) à HCl (aq) + HClO (aq)
It can be seen that chlorine starts with an oxidation state of zero. During the reaction it takes an oxidation state of -1 in hydrochloric acid and an oxidation state of +1 in chloric (I) acid.
Bacteria in the water are killed by free radical oxygen atoms by the slow decomposition of the chloric (I) acid:
HClO à HCl + ∙O
GLOSSARY
Disproportionation: a type of redox reaction in which the same species is both reduced and oxidised. It can be thought of as a self-reduction-oxidation reaction.
Sublimes: when a substance turns directly from a solid into a gas without passing through the liquid phase.
Van der Waals’ forces: the weak forces of attraction between molecules based on instantaneous and induced dipoles.