ATOMIC STRUCTURE
I) DISCOVERING THE ELECTRON
All known materials can be broken down into fundamental substances called elements.
Most elements exist in combinations with other elements in compounds, but some are found in an uncombined state.
When electricity flows in an aqueous solution of silver nitrate, for example, silver metal is deposited at the cathode. This is electrolysis.
The best explanation is that:
-
The silver exists in the solution as positively charged ions (Ag+).
-
One silver ion plus a unit of electricity gives an atom of silver.
This unit of electricity is called the electron.
-
William Crookes first investigated the effects of passing electricity through gases at low pressures in a cathode ray tube. He found that the containing vessel opposite the cathode glowed hot when a high voltage was applied. If a solid object was placed between the cathode and the screen, a shadow would be seen. Initially it was thought that this was caused by rays, however it was observed that the movement of a magnet deflected the ‘ray’. This is best explained by assuming the ‘ray’ is a stream of negatively charged particles due to the deflection direction in a magnetic field.
-
J.J. Thomson’s study of the effect of magnetic and electric fields upon electron beams allowed the charge to mass ratio of the electron to be calculated. They were found to have a mass of 1/2000th of a hydrogen atom.
-
Millikan’s oil drop experiment was the first to measure the electronic charge and electron’s mass.
The mass of the electron relative to the proton is 1/1837th.
II) DISCOVERY OF PROTONS AND NEUTRONS
J.J Thompson’s ‘plum pudding’ model of an atom consisted of electrons embedded in a ‘pudding’ of uniform positive charge.
Positive nucleus
The research of Hans Geiger and Ernest Marsden on alpha particles fired at metals led to a change of the atomic model. Whilst they detected small scattering effects expected from the interactions between positive charges and electrons, a few large deflections were also observed. Ernest Rutherford’s research team’s findings led to his proposition of a new nuclear model, consisting of a concentration of mass and positive charge in a very small nucleus, surrounded by mostly empty space and electrons.
Protons
In 1913, Henry Mosely found a way of comparing the positive charges of atoms of different elements. The charge increases by one unit from element to element in the periodic table. Mosely showed the pattern in properties is caused by atomic (proton) number rather than relative atomic mass.
By firing alpha particles through hydrogen, nitrogen and other materials, new particles called protons were isolated. Found to have the equal size but opposite sign to the electron, the mass was found to be 1.673 x 10^-27 kg, roughly 2000 times heavier than the electron.
Neutrons
Due to the charge of zero on a neutron, it doesn’t interact with any electrical or magnetic fields, making detection difficult. It was not until 1932 that one of Rutherford’s co-workers, James Chadwick produced sufficient evidence for the similarly massive but neutral particle, now known as the neutron.
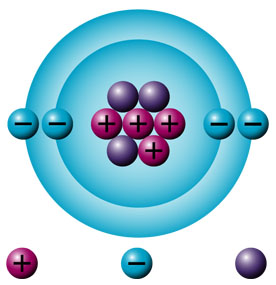
III) ATOMIC AND MASS NUMBERS
Atomic number (Z) is the number of protons in an atom. It is the atomic number of an atom which determines which element it is. the atomic number shows:
-
The number of protons in the nucleus of an atom of that element
-
The number of electrons in a neutral atom of that element
-
The position of the element in the Periodic Table.
Mass number (A) is the measure of the total number of particles in the nucleus of an atom.
for any atom, the mass number is the sum of the number of protons an number of neutrons.
Particle name Relative mass Relative charge Electron Negligible (about 1/2000th the mass of a proton) -1 Proton 1 1 Neutron 1 0
IV) ISOTOPES
Atoms of the same element are not all identical. It was proposed that atoms of the same element could have differing atomic masses due to extra or fewer neutrons. These atoms are called isotopes.
The discovery of protons and neutrons explained the existence of isotopes.
Elemental isotopes must have the same number of protons but differ in the number of neutrons.
-
Number of protons = Z
-
Number of neutrons = A- Z
-
Number of electrons in a neutral atom = Z
-
Number of electrons in an ion = Z - charge on ion.
V) COUNTING ATOMS AND MOLECULES
The relative atomic mass of an element is:
The average mass of the element relative to the mass of carbon-12 where one atom of this isotope is given a relative isotopic mass of exactly 12.
The relative atomic mass of an element is an average mass as it takes into account their relative abundance.
To calculate the relative atomic mass of an element, the relative isotopic mass number and the relative abundance of each isotope of an element must be known.
Relative isotopic mass is the mass of an isotope of an element relative to the mass of carbon-12, where 1 atom of carbon-12 is given an isotopic mass of exactly 12.
The relative molecular mass of a compound is the mass of a molecule of a compound relative to carbon-12, where an atom of carbon-12 is given a mass of exactly 12.
To find the relative molecular mass of a molecule, we find the sum of all the relative atomic masses of atoms present in the molecule.
Relative formula mass is the term used to mean relative molecular mass for compounds containing ions, whose formulae are given as ratios.
GLOSSARY
Atomic number: the number of protons in the nucleus of each atom of an element.
Compound: a substance made up of two or more elements chemically joined together.
Electrons: tiny negatively charged sub-atomic particles, found in orbitals around the nucleus of an atom.
Elements: substances made up of only one type of atom. The elements are listed in the Periodic Table.
Ion: a positively or negatively charged atom or (covalently bonded) group of atoms.
Isotopes: atoms of an element with the same number of protons but different numbers of neutrons.
Mass number: the total number of protons and neutrons in the nucleus of an atom.
Neutron: a sub-atomic particle found in the nucleus of an atom. It carries no charge and has the same mass as a proton.
Nucleus: the small, dense core at the centre of an atom, containing protons and neutrons (hence a nucleus is always positively charged).
Protons: positively charged sub-atomic particles, found in the nucleus of an atom
Relative atomic mass: the weighted average mass of the atoms of an element taking into account the relative abundance of its naturally occurring isotopes, measured on a scale on which carbon-12 is given a mass of exactly 12
Relative formula mass: the mass of one formula unit of a compound relative to an atom of carbon-12
Relative isotopic mass: the mass of an isotope of an atom of an element relative to an atom of carbon-12
Relative molecular mass: the mass of a molecule of a compound relative to an atom of carbon-12