ELECTRON STRUCTURE
I) ELECTRONS IN ATOMS
Electrons are fundamentally important in chemistry, as they are the causes of the changes observed in chemical reactions.
If we knew everything about the way electrons behave, we could predict the way chemicals behaved purely from mathematics.
Niels Bohr proposed that electrons only had in energy in quanta (small packets of energy) so could they could not exist anywhere outside the nucleus unless they were in fixed energy levels.
These energy levels are known as shells. Shells are numbered 1, 2, 3, 4, etc. These numbers are called principle quantum numbers (n).
In n = 1, up to 2 e-
In n = 2, up to 8 e-
In n = 3, up to 16 e-
In n = 4, up to 32 e-
Each shell (principle quantum number) is then split up into a number of subshells, which contain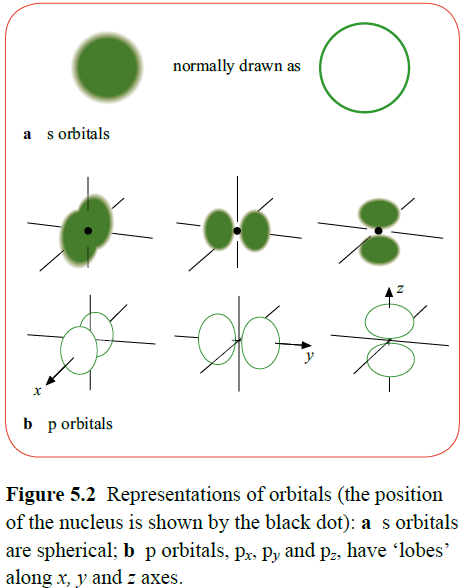 orbitals with different energy values.
orbitals with different energy values.
The subshells are labelled s, d, p and f. An s subshell contains one orbital, a p subshell contains three orbitals, a d subshell contains five orbitals and an f subshell contains seven orbitals.
An electron orbital represents a region of space around the nucleus of an atom, capable of taking two electrons. Therefore an s subshell holds two electrons, a p subshell six electrons, a d subshell ten electrons and an f subshell can accommodate fourteen electrons.
Each orbital has an approximate three dimensional shape. These are only approximations as they do not have exact boundaries, and so are fuzzy cloud shapes.
Any individual orbital can hold one or two electrons, but no more.
Two electrons can be held in one orbital despite their identical negative charges due to spin pairing. Two electrons in an orbital will both have opposite spin directions to reduce the effect of repulsion.
When filling electron shells and orbitals, the electrons fill the lowest energy levels first to give an energy state as low as possible.
II) IONISATION ENERGY
When an atom loses an electron it becomes a positive ion – the atom has been ionised. The amount of energy required to remove the electron is called its ionisation energy.
The first ionisation energy of an element is the amount of energy required to remove one electron from each atom in a mole of atoms of an element in its gaseous state.
Ionisation energy’s symbol is ΔHi, and the first ionisation is ΔHi1. The process is shown as a half equation, with the atom before ionisation on the left and the new ion and electron lost shown on the left. Calcium’s first ionisation energy is shown below:
Ca (g) à Ca+ (g) + e- ΔHi1 = + 590 kJ mol-1
The second ionisation energy is the amount of energy required to remove a further electron from each ion in a mole of gaseous ions.
Ca+ (g) à Ca2+ (g) + e- ΔHi2 = + 1150 kJ mol-1
Electrons can be continually removed until just the nucleus of an atom is left. For any one element it can be seen that:
-
Successive ionisation energies increase. As each electron is removed from an atom, the remaining ion becomes more positively charged. There is a stronger nuclear attraction which means more energy is required to remove an electron from an increasingly positive ion.
-
There are a number of particularly large rises within a set of ionisation energies.
Ionisation energies can be influenced by:
-
The size of the positive nuclear charge – as atomic number increases, nuclear charge is increased, as is the nuclear attraction. Therefore more energy required to remove electrons.
-
The distance of the electron from the nucleus (atomic radii increase) – all forces of attraction decrease rapidly as the distance between the attracted bodies increase. Thus the attraction is less strong on electrons further from the nucleus and stronger on those closer. The further a shell is from the nucleus, the weaker the electron-nuclear attraction. This overcomes the nuclear charge effect down groups. Therefore lower first ionisation energy
-
The shielding effect of inner shell electrons – electrons are negatively charged and so repel each other. Electrons in the inner shells repel outer shell electrons, increasing atomic radii and shielding them from the nuclear attraction. Therefore lower first ionisation energy
-
New subshell – a p subshell is of a higher energy than an s subshell. Therefore electrons in the p subshell will require less energy to remove as they already have more energy (causing decrease between group 2 and 3).
-
Spin pairing – in some cases (e.g. nitrogen and oxygen) it is easier to remove an electron from a fully filled orbital than a half filled orbital, due to the repulsion between electrons.
Ionisation energies can indicate which group an element is in: for group n, greatest increase between n and n + 1.
III) ELECTRONIC CONFIGURATIONS
Electron configurations are represented as shown below. For example, hydrogen has one electron in the s subshell in the shell with principle quantum number n = 1. This is shown as:
1s1
The superscript shows the number of electrons in the 1s subshell. The configuration for Helium is 1s2 as the 1s subshell contains two electrons.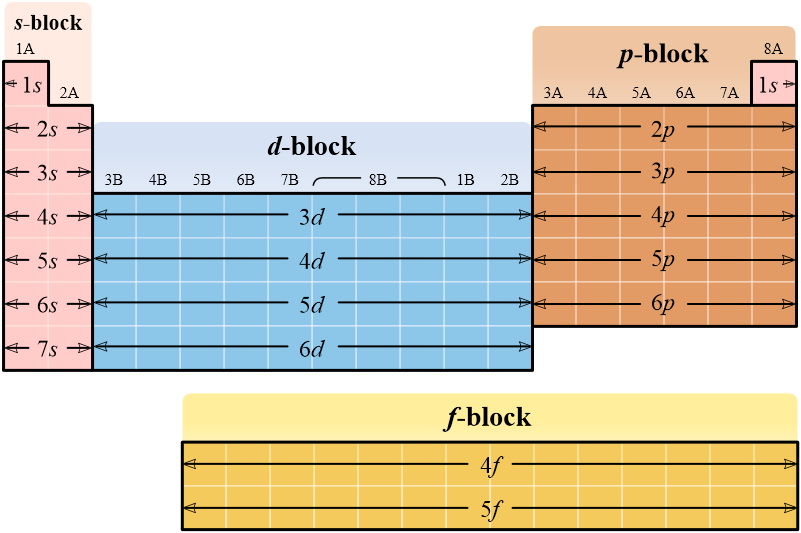
The electron configurations of atoms can be easily determined by the shape of the periodic table. Element which lie in the s-block have their last orbital in an s subshell.
For larger atoms with more complex configurations, the noble gas in the previous period is used to abbreviate the configuration of the electrons in previous quantum numbers.
Electron orbitals are filled in the order specified in the table.
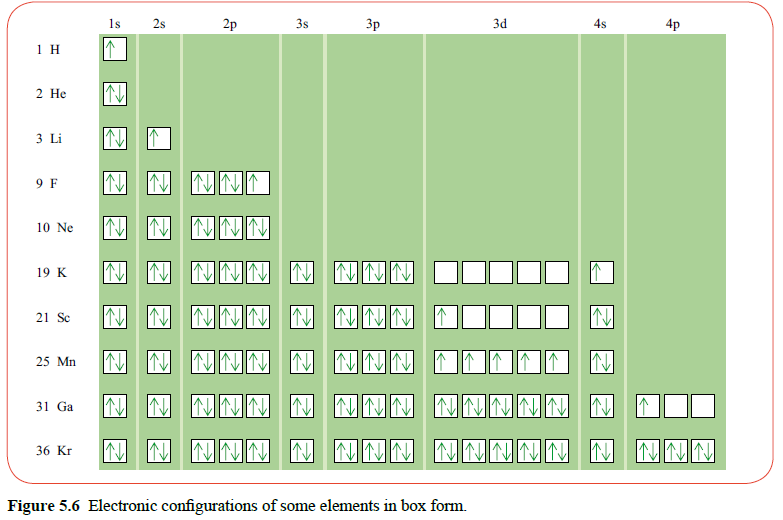 Electrons half fill each orbital in a subshell before fully filling them, as non-spin pairing orbitals are more stable than spin paired orbitals.
Electrons half fill each orbital in a subshell before fully filling them, as non-spin pairing orbitals are more stable than spin paired orbitals.
There are anomalous electron configurations however.
Chromium has configuration: [Ar] 4s1 3d5 Copper has configuration: [Ar] 4s1 3d10
As can be seen, an electron in the 4s subshell is promoted to the 3d subshell as half-filled subshells (and orbitals) are more stable than a fully filled subshell and a partially-filled subshell.
When atoms lose or gain electrons, the electrons are taken or filled from the highest energy level subshell. This effects their electronic configuration.
GLOSSARY
D-block elements: a block of elements found between Groups 2 and 3 in the periodic table.
Ionisation energy: the first ionisation is the energy needed to remove one electron from each atom in one mole of gaseous atoms or ions of an element.
Successive ionisation energies: the sequence of first, second, third, fourth, etc. ionisation energies needed to remove the first, second, third or fourth electrons from each atom in one mole of gaseous atoms of an element.
Transition elements: elements in the d-block that can form at least one ion with a partially filled d subshell.