BONDING AND STRUCTURE
I) IONIC BONDING
Ionic bonding results from the electrostatic attraction between the oppositely charged ions. In sodium chloride, the ions are arranged in a giant crystal lattice. The arrangement of ions in a lattice determines the shape and colour of the crystals.
As ionic compounds can conduct electricity when in aqueous solution or molten, they can be electrolysed (decomposed by electricity). Electrolysis is used, for example, to produce chlorine gas from salt water.
Ions are free to move through the aqueous solution or molten compound and are attracted to the oppositely charged electrode.
-
Positive ions (cations) are attracted to the negative electrode (cathode).
-
Negative ions (anions) are attracted to the positive electrode (anode).
At the cathode, electrons are donated to the positive ions. At the anode, electrons are taken from the negatively charged ions. When they return to their non-ionised state, they are elements. This causes metals to collect on the cathode and non-metals to form near the anode.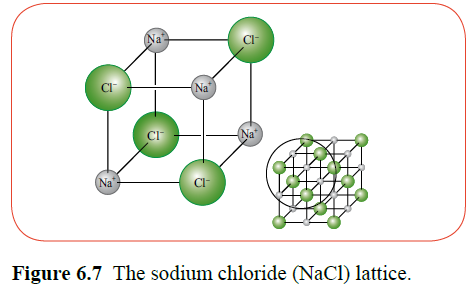
These changes are represented by separate half equations. With the example of electrolysis of NaCl solution:
2Cl- à Cl2 + 2e-
Na+ + e- à Na
Positive ions are formed when metals (and ammonia) lose electrons to achieve a full outer shell.
Negative ions are formed when non-metals gain electrons to achieve a full outer shell.
Dot and cross diagrams can be used to show the movement of electrons in the formation of ions. Only the outer shell is usually drawn.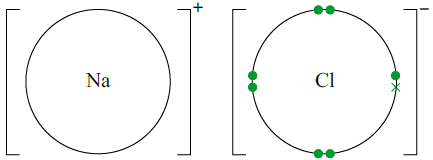
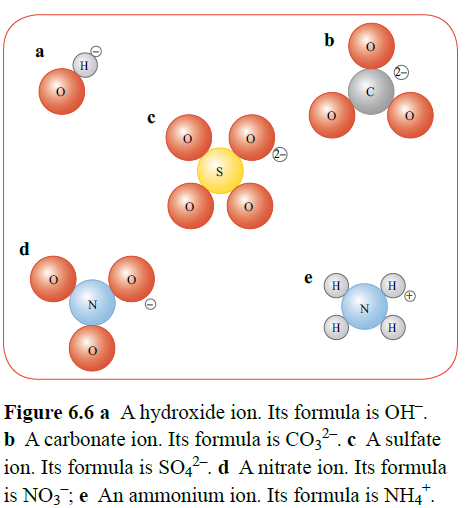
Compound ions consist of a small group of atoms, held together by covalent bonds to give an overall electric charge. This is due to the elements oxidation numbers.
Properties of ionic compounds
-
Ionic compounds are crystalline solids with high melting points.
- They conduct electricity when in aqueous solution or when molten – the passing of electric current makes them decompose in a process called electrolysis.
- They are hard and brittle however often easily dissolve in water.
Ionic compounds have a crystalline structure. Ionic bonds are very strong and so a large amount of energy is required to break the bonds. This leads to a high melting and boiling point of the compound.
The high melting point of ionic compounds makes them particularly useful.
Ionic compounds may dissolve in water. This is due to the polar nature of water and the polar nature of ionic compounds.
As a rule, most metal nitrates and metal halides are soluble. Ions that carry higher charges tend to be less soluble.
Group 1 hydroxides are soluble and group 2 hydroxides are sparingly soluble.
Sometimes water molecules become integrated as part of the crystalline structure, in a hydrated compound.
Common ion charges
|
Positive ion |
Charge on ion |
Negative ion |
Charge on ion |
|
H |
+ |
F / Cl /Br / I |
- |
|
Li / Na / K |
+ |
O / S |
2- |
|
Ca / Mg / Sr |
2+ |
SO4 |
2- |
|
B / Al |
3+ |
SO3 |
2- |
|
Cu (II) |
2+ |
NO3 |
- |
|
Fe (II) |
2+ |
NO2 |
- |
|
Fe (III) |
3+ |
CO3 |
2- |
|
Ag |
+ |
HCO3 |
- |
|
Zn |
2+ |
OH |
- |
|
NH4 |
+ |
|
|
II) COVALENT BONDING
Most familiar compounds are liquids or gases or solids with low melting points.
Such compounds have very different properties to ionic compounds, as they contain molecules – groups of atoms he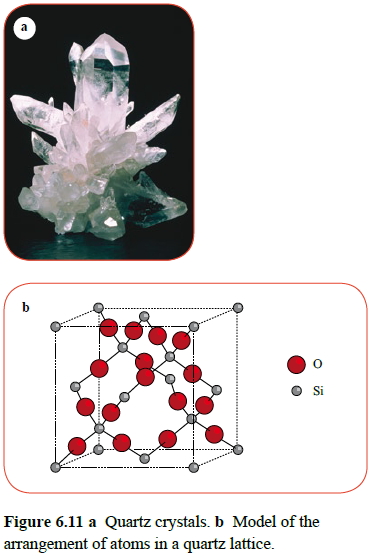 ld together by covalent bonds. They are said to have a simple molecular structure.
ld together by covalent bonds. They are said to have a simple molecular structure.
They are non-conductors of electricity and are may be insoluble in water. They may dissolve in organic solvents such as ethanol or cyclohexane.
On the other hand there are some crystalline covalent compounds that have very high melting points. In these cases, the covalent bonds extend throughout the crystal in a giant lattice structure, not as small molecules. An example would be SiO2, which forms crystals in a giant covalent structure.
A single covalent bond is a shared pair of electrons. Most covalent bonds are single bonds, consisting of one of the pair of electrons being from each atom.
Atoms can also bond together by sharing two pairs of electrons in a double covalent bond, such as in an O2 molecule.
Atoms can also form triple covalent bonds, such as in an N2 molecule.
Atoms in molecules frequently have pairs of electrons in their outer shells that are not involved in c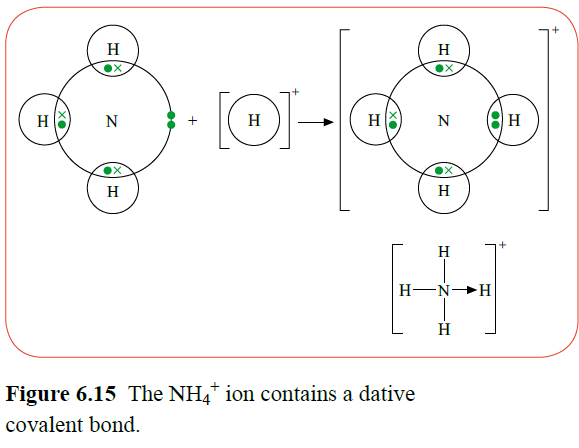 ovalent bonds. These non-bonding pairs are called lone pairs.
ovalent bonds. These non-bonding pairs are called lone pairs.
A lone pair can sometimes be used to form a covalent bond to an atom that can accommodate two more electrons in its outer shell. When the ammonia molecule and an H+ ion combine to form an ammonium ion, a dative covalent bond is formed.
During this bonding, the pair of electrons came from the nitrogen atom. The lone pair of the ammonia molecule has formed this bond, called a dative covalent bond.
Polar molecules
The electrons in a covalent bond may, on average, spend their time nearer to one of the two bonded atoms. That atom gains a small negative charge (δ-) and the other atom a small positive charge (δ+). The bond is said to be polar.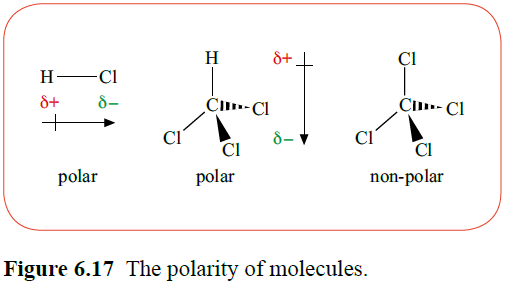
Polar covalent bonds are caused by a difference in the electronegativity of elements. Electronegativity is the tendency of a bonded atom to attract the electrons in the covalent bond towards itself.
Electronegativity generally increases across periods and vertically up groups.
Non symmetrical molecules with fluorine, oxygen, nitrogen or chlorine usually form dipoles, as these elements are most electronegative. In symmetrical molecules however, the presence of strongly electronegative atoms will cause the dipoles to cancel each other out.
Bond polarity can be an indicator of the reactivity of a molecule. Nitrogen and carbon monoxide both contain a triple bond. Nitrogen is a very unreactive molecule and is a non-polar molecule. Carbon monoxide is a very reactive molecule and is polar molecule due to oxygen’s electronegativity.
III) SHAPES OF SIMPLE MOLECULES
As electrons are negatively charged, they repel each other. An electron pair in the outer shell of the central atom of a simple molecule will exert a repulsion on the other lone pairs. This causes them to move as far apart as possible within the confines of the bonds between atoms in a molecule. This determines the three-dimensional shape of a molecule.
Lone pairs are pulled closer to the nucleus than bonding pairs, as bonding pairs are pulled towards two nuclei. The electron charge-cloud in the lone pair has a greater width than a bonding pair.
LP-LP repulsion > LP-BP repulsion > BP-BP repulsion
The variation in in repulsion produced effects on the bond angles in molecules.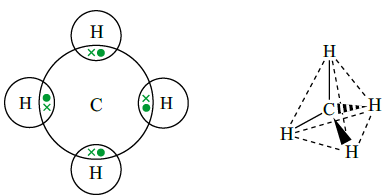
Methane
As carbon is tetravalent, it can form four covalent bonds in a molecule. The four bonding pairs of electrons repel each other towards the corners of a regular tetrahedron, causing the molecule to have a tetrahedral shape. Bond angles are 109.5° .
Ammonia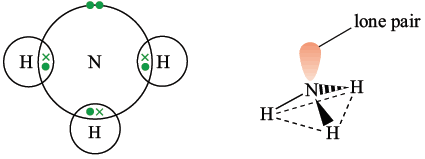
Ammonia has three bonding pairs and one lone pair on the central atom. The four electron pairs repel each other and occupy the corners of a tetrahedron, however the nitrogen and three hydrogen atoms form a triangular pyramidal. Bond angles are 107°.
Water
There are two bonding pairs and two lone pairs on the oxygen atom. Again these repel each other towards the corners of a tetrahedron, leaving the oxygen and two hydrogen atoms as a non-linear molecule. Bond angles are 104.5°.
Carbon dioxide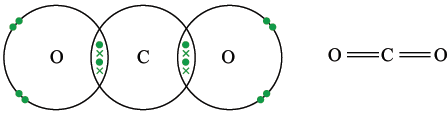
There are two carbon-oxygen double bonds. If the double bonds are considered in the same way as single electron pairs, they repel each other as far of possible, giving a linear shaped molecule. Bond angles are 180°.
Boron trifluoride
In this molecule, boron only has six electrons in its outer shell, distributed between three bonding pairs. The three bonding pairs repel each other equally, forming a trigonal planar molecule with bond angles of 120°. Boron is very reactive and will accept a lone pair of electrons from another molecule (dative covalent bond).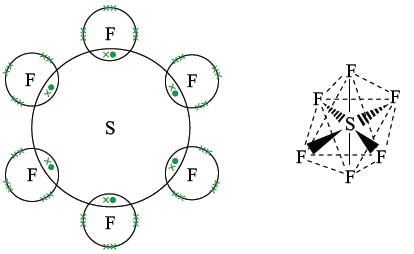
Sulphur hexafluoride
There are six bonding pairs and no lone pairs. Repulsion between six electron pairs causes the bonding electrons to be repelled as far away as possible, forming an octahedral shape. Bond angles are 90°.
Bond enthalpy is the energy required to break one mole of the given bond in the gaseous molecule.
IV) METALLIC BONDING
Metals have very different properties to both ionic and covalent compounds. In appearance they are usually lustrous.
They are good conductors of both heat and electricity. They conduct electricity in the solid state and are not decomposed by the passing current, unlike ionic compounds.
They are malleable and ductile, they often possess high tensile strengths and they are generally hard.
Metallic bonding is the attraction between positively charged metal ions (cations) and a ‘sea’ of delocalised conduction electrons, arranged in a lattice structure.
The sea of conduction electrons means that when an potential difference is applied, the electrons can freely move and so carry a charge (create a current).
Conduction of heat occurs by the vibration of the positive ions as well as by the mobile electrons.
Metals are both ductile and malleable because the bonding in the lattice is not broken when they are physically deformed. This is because the ions can freely slide over other when forces are applied.
Metals generally have high melting points as the attractions between the electrons and ions are strong, therefore a large amount of energy is required to dissociate them.
V) INTERMOLECULAR FORCES
Substances with simple molecular structures generally have low melting and boiling points.
Weak attractive forces, called intermolecular forces exist between molecules. These differ from the strong forces within molecules, such as covalent bonds.
The state at room temperature and pressure in simple molecular substances is due to the strength of intermolecular forces.
-
Substances with the weakest intermolecular forces have the lowest boiling points (so they are gases at r.t.p.) and substances with the strongest intermolecular forces have higher boiling points (they could be solid at r.t.p. like in iodine).
Therefore the intermolecular attractions are responsible for the states of matter in simple molecular substances.
There are three main types of intermolecular forces: van der Waals forces (instantaneously induced dipole-induced dipole attraction), dipole-dipole forces and hydrogen bonds.
Van der Waals forces
The forces arise because the electrons in atoms or molecules can be thought to be moving at very high speeds in orbitals.
-
At any instant time, it is possible for more electrons to be on one side of the atom or molecule than the other. This creates an instantaneous induced dipole.
-
The momentary imbalance of one molecule or atom causes the electrons on neighbouring atoms or molecules to be repelled, inducing a dipole.
-
The attraction between the instantaneous induced dipole and the induced dipole is what causes the intermolecular force.
The number of electrons and protons present influences the strength of van der Waals forces. In the halogens, as the atoms increase in size, the greater the chance a dipole is induced and the more electrons can be repelled, causing a greater difference in charge. The stronger the intermolecular force, the more energy is required to break the attractions (thus higher melting/boiling point).
Van der Waals forces are the weakest type of intermolecular force. They are responsible for the slippery nature of graphite, in contrast to the hardness of diamond.
Dipole-dipole forces
Water molecules have a permanent electric dipole.
Dipole-dipole forces are attractions between two permanently polar molecules, such as hydrogen chloride or water.
Hydrogen bonds
Water has quite a few anomalous properties:
-
The boiling point of water is much higher than predicted by the trend in group 6 hydrides.
-
Water also has a high surface tension and a high viscosity.
-
The density of ice is less than that of liquid water. Most solids are denser than liquids, as molecules usually pack closer together in solids than in liquids.
The peculiar nature of water is because water forms hydrogen bonds between its molecules, the strongest type of intermolecular force.
Water is highly polar due to the difference in electronegativity between oxygen and hydrogen. The resulting intermolecular attraction between hydrogen of one molecule and oxygen of another is a very strong dipole-dipole attraction called a hydrogen bond.
Each water molecule can form up to two hydrogen bonds. These form in the directions of the lone pairs.
-
In the liquid state, water molecules collect in groups. On vaporisation, the hydrogen bonds must be broken. This raises the boiling point of water significantly as hydrogen bonds are stronger than other intermolecular forces.
-
In ice, the hydrogen bonds make the water molecules form a crystal shaped lattice. In this lattice, each oxygen atom is surrounded by a tetrahedron of hydrogen atoms bonded to further oxygen atoms. The network of hydrogen bonds raises the freezing point of water compared to other group 6 hydrides.
Water’s high surface tension is caused by the presence of a hydrogen bonded network on the surface. The network is sufficiently strong to hold very light objects upon it.
Hydrogen bonds also form between ammonia molecules and play a vital role in the structure of DNA base pairs.
GLOSSARY
Bond enthalpy: the amount of energy needed to break one mole of a particular bond in one mole of gaseous molecules.
Dative covalent bond: a covalent bond where both electrons come from one atom.
Double covalent bond: two shared pairs of electrons that bond two atoms together.
Electrolysis: the decomposition of a substance caused by the passing of a d.c. electric current.
Electronegativity: describes the ability of an atom to attract the bonding electrons in a covalent bond.
Hydrogen bond: a weak intermolecular bond formed between molecules containing a hydrogen atom bonded to one of the most electronegative elements (N, O or F).
Ionic bonding: the electrostatic attraction between positively charged ions.
Polar covalent bond: a covalent bond in which the two bonding electrons are not shared equally by the two bonded atoms. The atom that gets the bigger share of the two electrons becomes δ-. The atom that gets the smaller share of the two electrons becomes δ+.
Single covalent bond: a shared pair of electrons that bonds two atoms together.
Triple covalent bond: three shared pairs of electrons that bond two atoms together.
Van der Waal’s forces: the weak forces of attraction between molecules based on instantaneous and induced dipoles.