ACIDS
I) ACIDS AND BASES
When acids are put into water, they dissociate. An acid is a compound that releases H+ ions (protons) in aqueous solution. An acid is a proton donor. A strong acid will dissociate fully into ions, whereas a weak acid will only partially dissociate.
Three common acids dissolving in water are shown as:
Hydrochloric acid HCl(g) à H+(aq) + Cl-(aq)
Sulphuric acid H2SO4 (l) à 2H+(aq) + SO42- (aq)
Nitric acid HNO3 (aq) à H+ (aq) + NO3- (aq)
A base is a substance that accepts H+ from an acid. Bases can neutralise acids. Metal oxides and hydroxides are bases, as is ammonia. Bases are proton acceptors.
Sodium hydroxide and potassium hydroxide are alkalis. An alkali is a soluble base. Alkalis release OH- ions in aqueous solution.
Sodium hydroxide dissolving in water is shown as:
NaOH (s) à Na+ (aq) + OH- (aq)
When ammonia gas dissolves in water, some of the ammonia molecules react with water molecules:
NH3 (g) + H2O (l) ↔ NH4+ (aq) + OH- (aq)
When a base neutralises an acid, the base accepts the H+ ions donated by the acid. At the same time, the H+ ions of the acid are replaced by metal ions or NH4+ ions, forming a salt.
If the base is a metal hydroxide, the OH- ions accept the H+ ions, forming water.
If the base is a metal oxide, the O2- ions accept the H+ ions, forming water also.
II) MAKING SALTS
A salt is any chemical compound formed from when an acid when it’s H+ ion is replaced by a metal ion or another positive ion, such as the ammonium ion NH4+.
Salts are made during neutralisation reactions as shown in Figure 3.2.
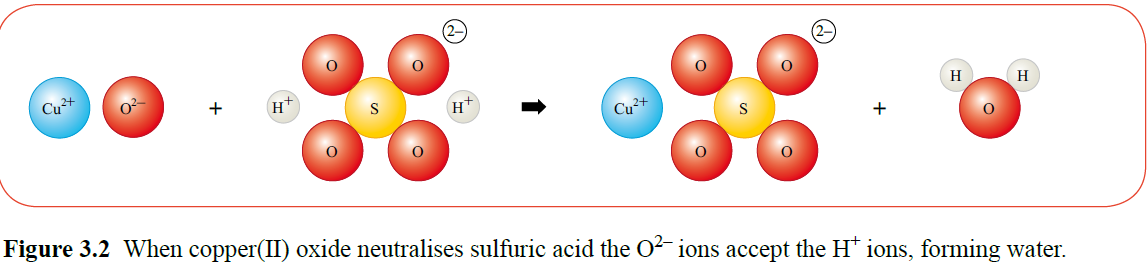
The salt in the reaction is copper sulphate. Adding excess copper oxide ensures that all of the sulphuric acid has been neutralised.
Salts can also be made by neutralising acids with metal carbonates. During the reaction, carbon dioxide, water and a salt is formed. Effervescence is seen and the solid carbonate dissolves into a soluble salt.
Titrations are examples of using neutralisation reactions in quantitative analysis.
For a titration, accurate measurements are very important. A calibrated pipette is used to accurately measure the 25 cm3 solution (usually the alkali) into the conical flask.
A burette is used to accurately measure the amount of solution which neutralises the solution in the flask.
The substance in the burette should be used to clean the burette before its use. Distilled water should be used to wash equipment between uses.
It is important that an indicator such as phenolphthalein or methyl orange is used as a sharp colour change is required to show when the solution is neutral (end point). A white tile makes the end point clearer.
The solution of salt at the end can be evaporated off, to leave the salt in solid form.
III) ANHYDROUS AND HYDRATED SALTS
Some salts, such as copper sulphate, have water crystals incorporated into their structure. These salts are said to be hydrated.
The water is known as water of crystallisation.
Hydrated copper(II) sulphate has a formula of CuSO4 ∙ 5H2O
The formula shows that there are 5 water molecules associated with each CuSO4 formula unit.
Copper sulphate can also exist without any water of crystallisation, where it said to be anhydrous.
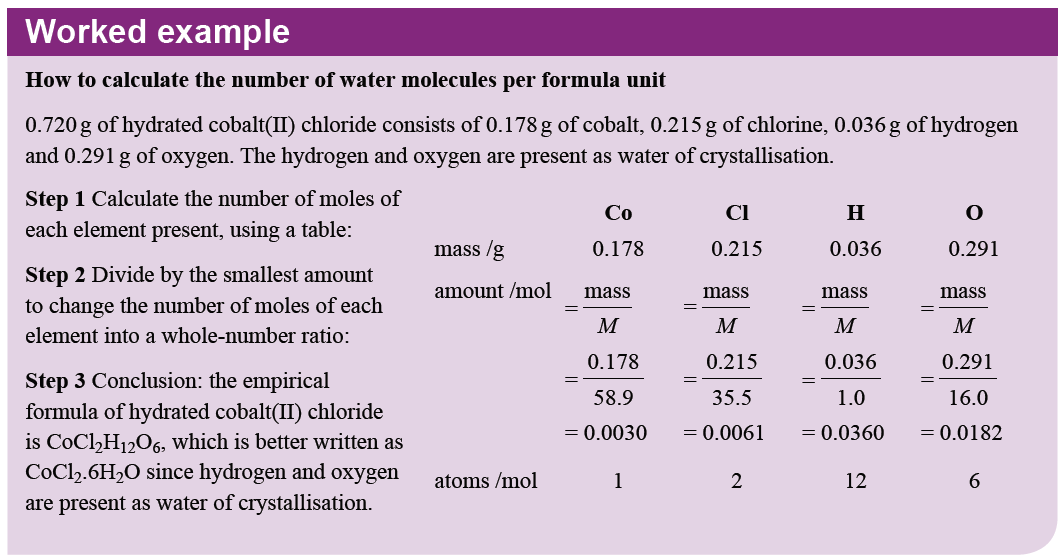
Similar to calculating empirical formulae, the ratio of salt to water of crystallisation can be calculated, as outlined below:
GLOSSARY
Acid: a chemical species which can donate a proton, H+. Strong acids dissociate fully into ions; weak acids only partially dissociate into ions.
Alkali: a soluble base that releases OH- ions in aqueous solution.
Anhydrous: without water of crystallisation.
Base: a substance that readily accepts a proton (H+) from an acid.
Hydrated: with water of crystallisation.
Salt: a compound produced when the H+ ion of an acid is replaced by a metal ion or an ammonium ion.
Titration: measurement of the exact amount of one solution needed to react with a fixed amount of another solution.
Water of crystallisation: water molecules incorporated into the crystal structure of a salt.